Do the Commission exercise their discretion under Article 37(5) CLP
The Commission has the inherent flexibility and the flexibility provided by the CLP Regulation how they incorporate RAC opinions into the ATP.
Article 37(5) of CLP (link)
Article 37(5) of the CLP spells out how the Commission are meant to take forward the incorporate new RAC opinions.
“The Commission shall without undue delay adopt delegated acts in accordance with Article 53a, where it finds that the harmonisation of the classification and labelling of the substance concerned is appropriate, to amend Annex VI by inclusion of that substance together with the relevant classification and labelling elements in Table 3.1 of Part 3 of Annex VI and, where appropriate, the specific concentration limits or M-factors. A corresponding entry shall be included in Table 3.2 of Part 3 of Annex VI subject to the same conditions, until 31 May 2015. Where, in the case of harmonisation of classification and labelling of substances, imperative grounds of urgency so require, the procedure provided for in Article 53b shall apply to delegated acts adopted pursuant to this paragraph” (emphasis added).
It is a discretion that the Commission use rarely exercise. Some deny that the discretion exists.
A recent case shows that the Commission does, although rarely, exercise their discretion.
Second Order Impacts
If the Commission can and do exercise this discretion it invokes second order impacts.
As a working rule the Commission have turned down requests to run impact assessments on classification proposals.
Their reasoning is that they have no discretion and their guidance provides them the margin of manouvre wheen they have discretion.
“1. An impact assessment will be necessary where there are likely to be significant impacts and where the Commission has discretion about the measures which could be taken (including whether to act at all). For example, a scientific body may recommend a safe exposure level to a particular chemical but the Commission has the choice of how best to manage the risks of exposure to that chemical’.
The Commission can’t pick and choose. If they decide in one case they have the discretiont to take forward a scientific body’s opinion, surely that discretion is open for all. To treat similar cases differently is poor governance.
Case Study – DTPA
In the case pentapotassium (DTPA) (CAS Number: 7216-95-7/ EC Number: EC Number: 404-290-3) the RAC’s Opinion of 9 June 2017 (link) recommended:
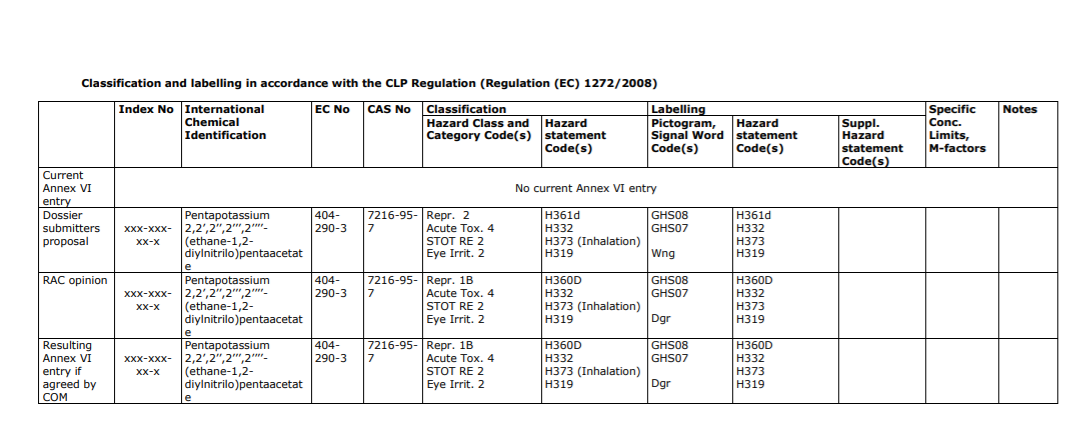
In the case of DTPA, an early draft of the 14th ATP agreed with the RAC.
14th Adaptation to Technical Progress (ATP), the new classification of DTPA-Na5 would be:
(1) Reproductive toxicity 1B; H360D;
(2) Acute toxicity 4; H332;
(3) STOT. RE 2; H373 (inhalation).
Later on it was amended. The 14th ATP (link) (as adopted by the Commission) removed the 1B listing and instead provided only for:
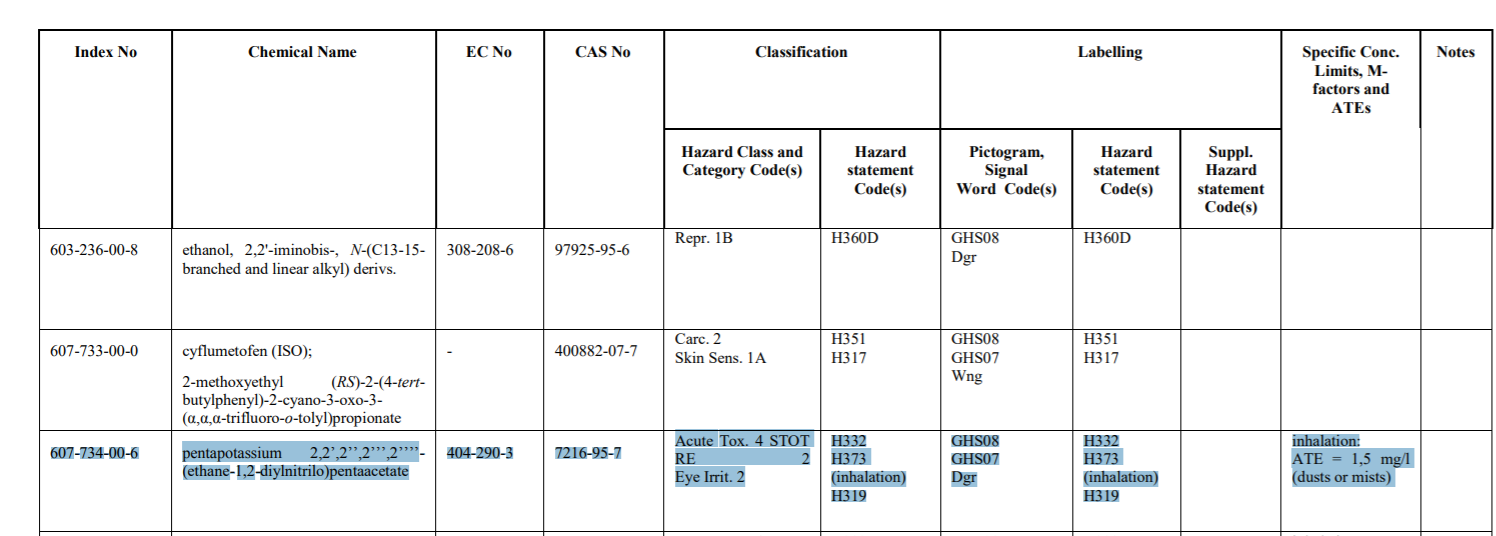
The recital explains the reason(s) for this change (link):
(6) With regard to the substances pentapotassium 2,2’,2’’,2’’’,2’’’’-(ethane-1,2-diylnitrilo)pentaacetate, N-carboxymethyliminobis(ethylenenitrilo)tetra(acetic acid) and pentasodium (carboxylatomethyl)iminobis(ethylenenitrilo)tetraacetate (DTPA), the classification as acute toxicant category 4 and specific target organ toxicant -repeated exposure (category 2) recommended in the RAC opinions of 9 June 2017 should be included in Annex VI to Regulation (EC) No 1272/2008, since sufficient scientific evidence is available justifying those new classifications. With regard to the substances pentapotassium 2,2’,2’’,2’’’,2’’’’-(ethane-1,2-diylnitrilo)pentaacetate and N-carboxymethyliminobis(ethylenenitrilo)tetra(acetic acid), the classification as eye irritant category 2, recommended in the RAC opinions of 9 June 2017, should be included in Annex VI to Regulation (EC) No 1272/2008, since sufficient scientific evidence is available justifying those new classifications. However, the classification of the substances pentapotassium 2,2’,2’’,2’’’,2’’’’-(ethane-1,2-diylnitrilo)pentaacetate, N-carboxymethyliminobis(ethylenenitrilo)tetra(acetic acid)and pentasodium (carboxylatomethyl)iminobis(ethylenenitrilo)tetraacetate (DTPA), as toxic for reproduction category 1B should not be included, since it requires further assessment by RAC in view of new scientific data on toxicity for reproduction presented by the industry after the RAC opinions were forwarded to the Commission.”(emphasis added).