This is a follow update of my review of the work of the Environment Committee’s scrutinising of delegated legislation in 2015. You can find that piece here.
Method
I have reviewed the agenda of each meeting of the Environment Committee in 2016. For each challenge to a delegated legislative proposal, I have tracked the success at the Committee and Plenary (via Vote Watch) stage.
I will update this blog when the Commission and European Parliament publish their annual activity reports.
Insights
Summary of Challenges by the Environment Committee in 2016
In 2016, the Environment Committee were notified of the following measures (these numbers need to be validated – there is ):
- 1521 Implementing Acts
- 27 Delegated Acts
- 366 RPS – Regulatory Procedures with Scrutiny measures
There is significant difference in the numbers reported on the Commission’s website. It reports(see here) 138 delegated acts in 2016 overall, 1494 implementing acts with committee control (comitology) and 123 RPS measures.
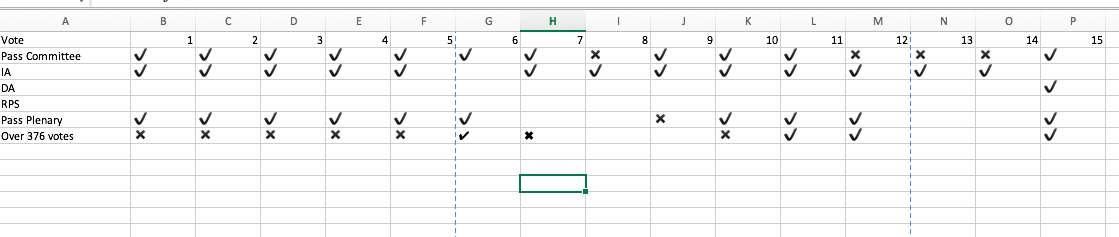
The European Parliament Environment Committee met 20 times in 2016. At 9 meetings there were 15 objections:
- 4 objections against pesticides
- 2 for insecticides
- 7 GMOs
- 4 regarding food products.
Even though the Environment Committee is active in scrutinizing the Commission’s delegated legislative output, the percentage of proposals they call in to challenge is very low. 15 objections out of 1914 proposed measures is a challenge rate of 0.7%. The idea that Environment Committee is “activist” on their scrutiny of comitology proposals is not borne out by the data.
1 objection amounted to a veto (see baby food – challenge 15).
The EP only has an effective veto on RPS measures and Delegated Acts. As most proposed measures are Implementing Acts, with the Parliament being unable to block a proposal, the Parliament have evolved their tactics. The glyphosate case showed the EP acting early to inform the Member State Committee before hand. This challenge led to, or was part of the reasons, that led to European Commission changing their original 15 years renewal to an 18 months technical extension with other conditions on use attached. The challenges against GMOs (implementing acts) led to no changes to the measures.
For delegated legislation,in all likelihood, whatever the Commission put out the door, will be adopted. Challenges are noticeable because they are so rare, successful challenges rarer.
In 2016, the Environment Committee focussed on a few areas: GMO, pesticides, and baby food. These are all sensitive areas of public policy so it is not a surprise that they receive scrutiny. In 2015, challenges also concerned other hazardous substances and broader food issues.
The EPP have little success in blocking proposals they have not supported in the Environment Committee. Rather, the decisive step appears to be when the S&D splits at the margins.
Resolutions launched by the ENF do not pass the Committee stage.
Single MEP initiatives do not have a good track record of success.
Many resolutions that succeed are launched as cross party efforts at the Committee stage and that coalition is kept going in the plenary.
Meetings in 2016
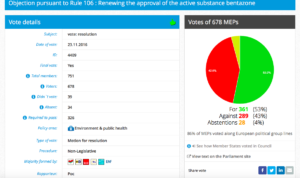
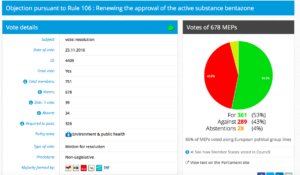
Challenge 1: Meeting 7 November 2016, 15.00 – 16.15. Item 7: Objection pursuant to Rule 106 : Renewing the approval of the active substance bentazone
Rapporteur: Pavel Poc (Czech/S&D)
Challenge: Implementing Act
Committee resolution here.
Decision in Committee: For 30, Against 21, Abstention 1.
Plenary Vote Watch summary
Date of vote: 23.11.2016
Resolution adopted here
Vote in Plenary: For 361, Against 289, Abstentions 28
Threshold: 326
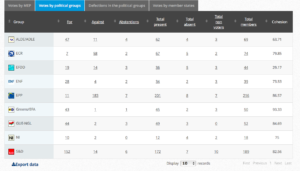
Coalition: GUE, Greens, S&D, ALDE, EFFD, ENF
Vote Watch Visual Summary
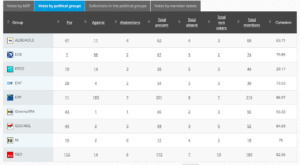
Voting by Group Summary
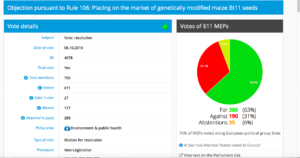
Challenge 2: Monday 3 October 2016 – Monday 3 October 2016; Item 6: Objection pursuant to Rule 106: Placing on the market for cultivation of genetically modified maize Bt11 (SYN-BTØ11-1) seeds
Co-Rapporteurs: Bart Staes (Belgium/ Greens/EFA), Guillaume Balas (France/S&D (, Lynn Boylan (Ireland/GUE/NGL), Eleonora Evi (Italy/EFDD) Sirpa Pietikäinen (Finland/EPP).
Committee resolution here
Decision in Committee Adopted: In favour: 37; Against: 18; abstention(s): 1.
Plenary Vote Watch Summary
Date of Vote: 6.10.2017
Resolution adopted here
Vote in Plenary: For 360, Against 190, Abstentions 35
Threshold: 289
Coalition: GUE, Greens, S&D, ALDE, EFFD, ENF
Vote Watch Visual Summary
Vote by Group Summary
Challenge 3: Monday 3 October 2016 – Monday 3 October 2016: Item 7. Objection pursuant to Rule 106: Placing on the market for cultivation of genetically modified maize 1507 (DAS-Ø15Ø7-1) seeds
Co-Rapporteurs: Bart Sates (Belgium/ Greens/EFA), Guillaume Balas (France/S&D (, Lynn Boylan (Ireland/GUE/NGL), Eleonora Evi (Italy/EFDD) Sirpa Pietikäinen (Finland/EPP).
Committee resolution here
Decision in Committee. Adopted: In favour: 39; Against: 17; Abstention(s): 0.
Plenary Vote Watch summary
Date: 6.10.2016
Resolution adopted here
Vote in Plenary: For 375; Against 193;Abstentions 36
Coalition: GUE, Greens, S&D, ECR, EFFD, ENF
Vote watch visual
Vote by Group Summary
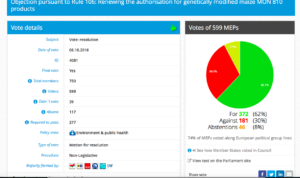
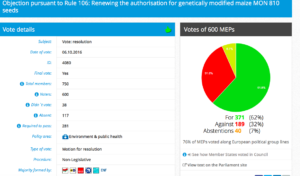
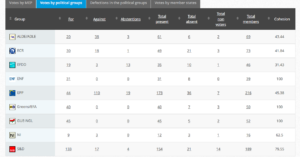
Challenge 4: Monday 3 October 2016 – Monday 3 October 2016: Objection pursuant to Rule 106: Renewing the authorization for the placing on the market for cultivation of genetically modified maize MON 810 (MON- ØØ81Ø-6) seeds
Co-Rapporteurs: Bart Staes (Belgium/ Greens/EFA), Guillaume Balas (France/S&D), Lynn Boylan (Ireland/GUE/NGL), Eleonora Evi (Italy/EFDD) Sirpa Pietikäinen (Finland/EPP).
Committee resolution here
Decision in Committee. Adopted: For: 37; Against: 19; abstention(s): 0.
Plenary Vote Watch summary here
Date of vote: 06.10.2016
Resolution adopted here.
Vote in Plenary: For 372; Against 181; Abstentions: 46
Threshold:277
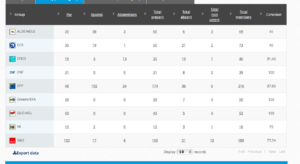
Coalition: GUE, Greens, S&D, ECR, EFFD, ENF
Vote Watch Summary
Vote by Group Summary
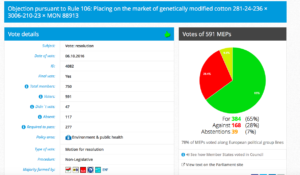
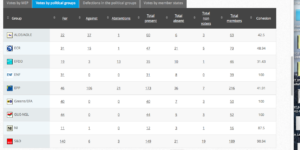
Challenge 5: Monday 3 October 2016 – Monday 3 October 2016: Objection pursuant to Rule 106: Placing on the market of products containing, consisting of, or produced from genetically modified cotton 281-24-236 × 3006- 210-23 × MON 88913 (DAS-24236-5×DAS-21Ø23-5×MON-88913-8)
Co-Rapporteurs: Bart Sates, Guillaume Balas, Lynn Boylan, Eleonora Evi Sirpa Pietikäinen.
Committee resolution here
Decision in Committee: For: 39; Against: 17;
Plenary Vote Watch summary here
Date of vote: 6.10.2016
Resolution adopted here
Vote: For 384, Against 169, Abstentions 39.
Threshold: 277
Coalition: GUE, Greens, S&D, ALDE, EFFD, ENF
Vote Watch Summary
Vote by Group Summary
Challenge 6: Monday 3 October 2016 – Monday 3 October 2016. Item 10 Objection pursuant to Rule 106: Renewing the authorisation for the placing on the market of genetically modified maize MON 810 (MON-ØØ81Ø-6) products
Co-Rapporteurs: Bart Staes, Guillaume Balas, Lynn Boylan, Eleonora Evi Sirpa Pietikäinen.
Committee resolution here
Decision in Committee. For: 30, Against: 19; abstention(s): 0.
Plenary Vote Watch summary here
Date of vote: 6.10.2016
Resolution adopted here
Vote: For 371, Against 189, Abstentions 40.
Threshold: 281
Coalition: GUE, Greens, S&D, EFFD, ENF
Vote Watch Summary
Vote by Group Summary
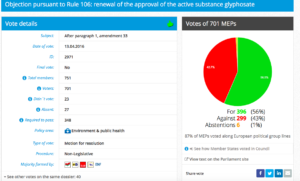
Challenge 6: DRAFT AGENDA – Monday 11 July 2016 – Tuesday 12 July 2016 Item 10Objection pursuant to Rule 106: extension of the approval period of glyphosate
Rapporteur: Merja Kyllönen (GUE/NGL
Adoption of motion for Resolution
Decision in Committee: Rejected. For: 9; Against: 28; abstention(s): 22.
Challenge 7. DRAFT AGENDA – Tuesday 21 June 2016 – Tuesday 21 June 2016. Item 8: Objection pursuant to Rule 106: Maximum residue levels for thiacloprid in or on certain products
Co-rapportuer: Sylvie Goddyn (ENF)
Decision in Committee: rejected: For: 7, Against: 41, Abstentions: 1
Challenge 8. DRAFT AGENDA – Wednesday 15 June 2016 – Thursday 16 June 2016. Item 6: Objection pursuant to Rule 106: Health claims on caffeine (Rule 106(2))
Rapporteur: Christel Schaldemose (S&D)
Committee Resolution here
Decision in Committee. Adopted. For: 57; Against: 0; Abstention(s):0
Plenary Vote Watch summary
Date: 07.07.2016
Resolution voted on here
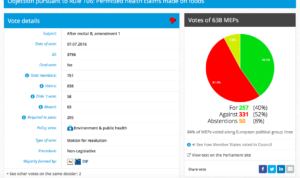
Defeated: For 257, Against 339, Abstenstions 50
Threshold 259
Coalition: EPP, ECR,ENF
Vote watch summary
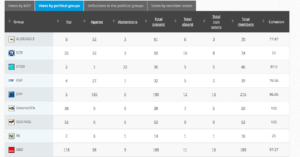
Voting by Group Summary
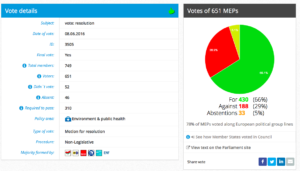
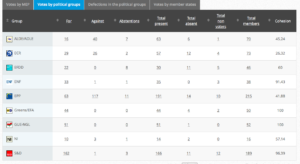
Challenge 9. DRAFT AGENDA – Monday 23 May 2016 – Tuesday 24 May 2016. Item 6: Objection pursuant to Rule 106: placing on the market of a genetically modified carnation (Dianthus caryophyllus L., line SHD-27531-4)
Co-Rapporteurs: Bart Staes, Lynn Boylan, Guillaume Balas. Adoption of motion for a resolution
Committee Resolution here
Decision: Adopted: Yes: 39; Against: 23; Abstention(s): 1.
Plenary Vote Watch summary
Date: 08.06.2016
Resolution adopted here.
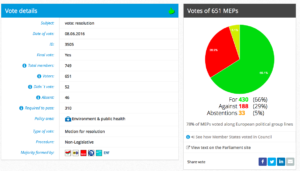
For 430, Against 189, Abstentions 33
Threshold 310
Coalition: GUE, Greens, S&D, EFFD, ENF
Vote watch Summary
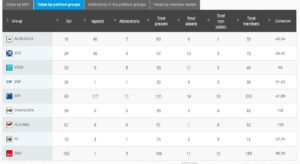
Voting by Group Summary
Challenge 10. DRAFT AGENDA – Monday 23 May 2016 – Tuesday 24 May 2016. Item 14: Objection pursuant to Rule 106: authorisation of GMO maize Bt11 x MIR162 x MIR604 x GA21
Co-Rapporteurs: Bart Staes, Lynn Boylan, Guillaume Balas.
Committee Resolution here.
Decision: Adopted:For: 39; Against: 24; Abstention(s): 0.
Plenary Vote Watch Summary
Date of vote: 8.06.2016
Vote in Plenary:For: 426; Against: 202; Abstensions: 33
Threshold: 315
Coalition: GUE, Greens, S&D, EFFD, ENF
Vote Watch Summary
Group Summary
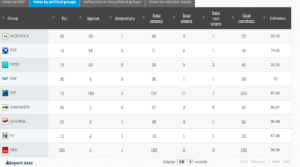
Challenge 11. DRAFT AGENDA – Monday 21 March 2016 – Tuesday 22 March 2016. Item 5: Objection pursuant to Rule 106: renewal of the approval of the active substance glyphosate
Co-Rapporteurs: Pavel Poc, Kateřina Konečná, Bas Eickhout, Piernicola Pedicini, Mark Demesmaeker, Sirpa Pietikäinen, Frédérique Ries
Committee Resolution here.
Decision in Committee. Adopted. For: 38; Against: 6; Abstention(s): 18
Plenary Vote Watch Summary
Date of vote: 13.04.2016
Resolution adopted here.
Vote in Plenary: For 396; Against 299 ;Abstenstions 6
Threshold: 348
Coalition:GUE, Greens, S&D, ALDE, ENF
Vote Watch Summary
Group Summary
Challenge 12. DRAFT AGENDA – Monday 21 March 2016 – Tuesday 22 March 2016. Item 12: Objection pursuant to Rule 106: renewal of the approval of the active substance glyphosate
Rapporteur: Mireille D’Ornano (ENF)
Committee Decision: Resolution Fell (see above challenge 11).
Challenge 13. Agenda Thursday 14 January 2016. Item 5: Objection pursuant to Rule 105: infant and follow-on formula
Rapporteur: Keith Taylor (Green/UK)
Committee resolution here
Decision in Committee: For 16; Against: 47; Abstention(s): 0
Challenge 14. Agenda Thursday 14 January 2016. Item 6: Objection pursuant to Rule 105: food for special medical purposes
Committee resolution here
Decision in Committee: 17; against: 46; abstention(s): 0
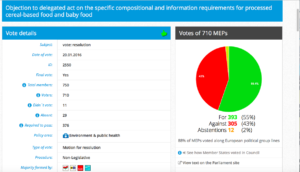
Challenge 15. Agenda Thursday 14 January 2016. Item 7. Objection pursuant to Rule 105: processed cereal-based food and baby food
Rapporteur: Keith Taylor (Green/UK)
Committee resolution here
Decision in Committee: For: 35; Against: 28; Abstention(s): 0
Plenary Vote Watch Summary
Date of Vote: 20.01.2016
Resolution adopted here
Vote in Plenary: For: 393; Against 305; Abstenstions: 12
Threshold: 376
Vote Watch Summary
Group Summary